Producer recalls saline product over 'stain'
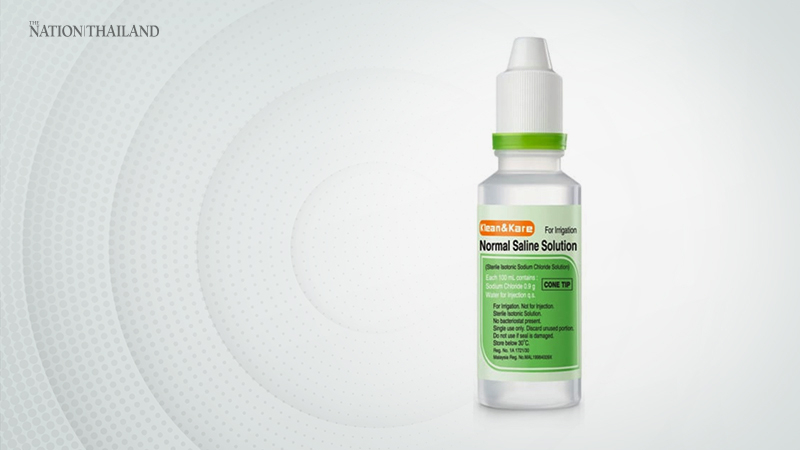
The Food and Drug Administration (FDA) said that the stain found on the bottle mouth of a Klean&Kare saline product at a hospital has not contaminated the liquid inside.
The agency’s statement came after Ramathibodi Hospital informed the FDA of the dust stain, FDA secretary-general Surachoke Tangwiwat said.
“The normal-saline lot number is 061607, produced in October 2019 by ANB Laboratories,” he said. “Moreover, we have not seen any stain or contamination in other saline bottles.”
The FDA informed the producer about the stain on February 6, and the producer decided to recall similar saline products from hospitals, pharmacies and other places.
Klean&Kare revealed the case on February 8 through its Facebook page, adding that “those who have the normal saline of the same lot can inform us so we can recall it”.
RELATED
