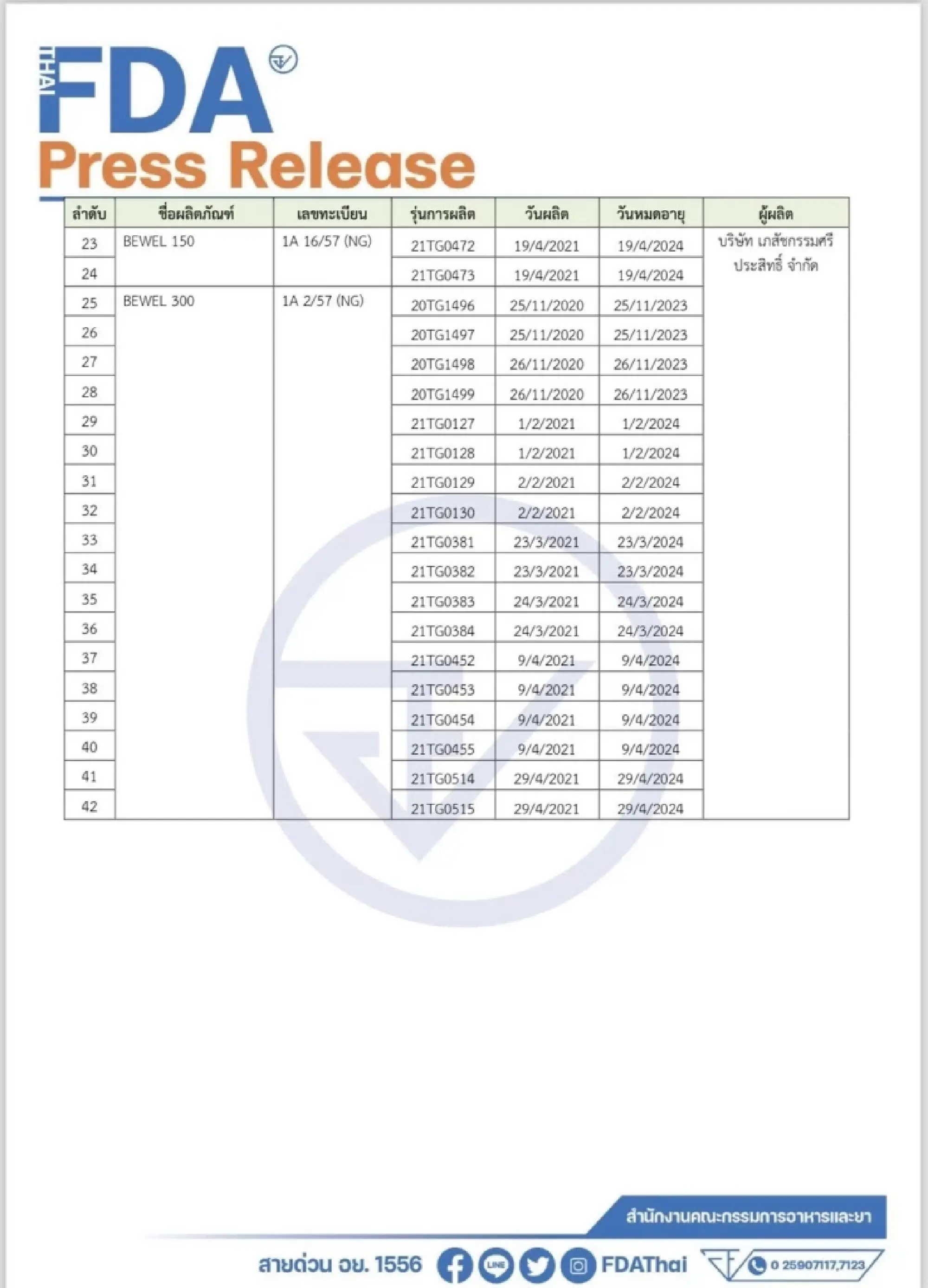
Thailand's Food and Drug Administration (FDA) issued a recall on 42 batches of Irbesartan, a drug used to treat high blood pressure, following a foreign report that a contamination of AZBT had been found in the drug ingredients.
AZBT, or azidomethyl biphenyl tetrazole, is a mutagen that may increase a patient's cancer risk over time.
Acting FDA secretary-general Dr Narong Aphikulvanich said on Friday investigation showed that the affected Irbesartan in Thailand were in 42 batches by five manufacturers, with details shown in the attached tables.
Irbesartan from different batches or different manufacturers can be safely consumed, he insisted.

He added that the FDA had thoroughly checked all manufacturers of the drug in the market and collected all samples for lab testing at the Department of Medical Science to make sure they were free of contaminants.
The FDA ordered the five manufacturers to recall all batches found to have been contaminated with AZBT, as well as alerted hospitals, clinics and pharmacies who had these drugs in their stock.
Narong added that patients who had been taking Irbesartan should not stop taking them immediately. They have been advised to check if the drug they are using is in the affected batches. If they are, contact physicians or pharmacists.
For more information, contact e-mail: [email protected] or tel. 02-590-7405

